Epitome Pharmaceuticals
5 April 2016
Introduction and the current situation: Precision medicine depends on tissue samples to determine DNA, RNA and protein characteristics of tumors and other tissue. We present an ideal scenario for tissue acquisition, handling and processing that may satisfy all parties. We have developed the scenario following discussions with interventional radiologists, laboratory personnel, study directors and others involved in precision medicine. We call this solution the “Right Sample,” as it satisfies the requirements of precision medicine.
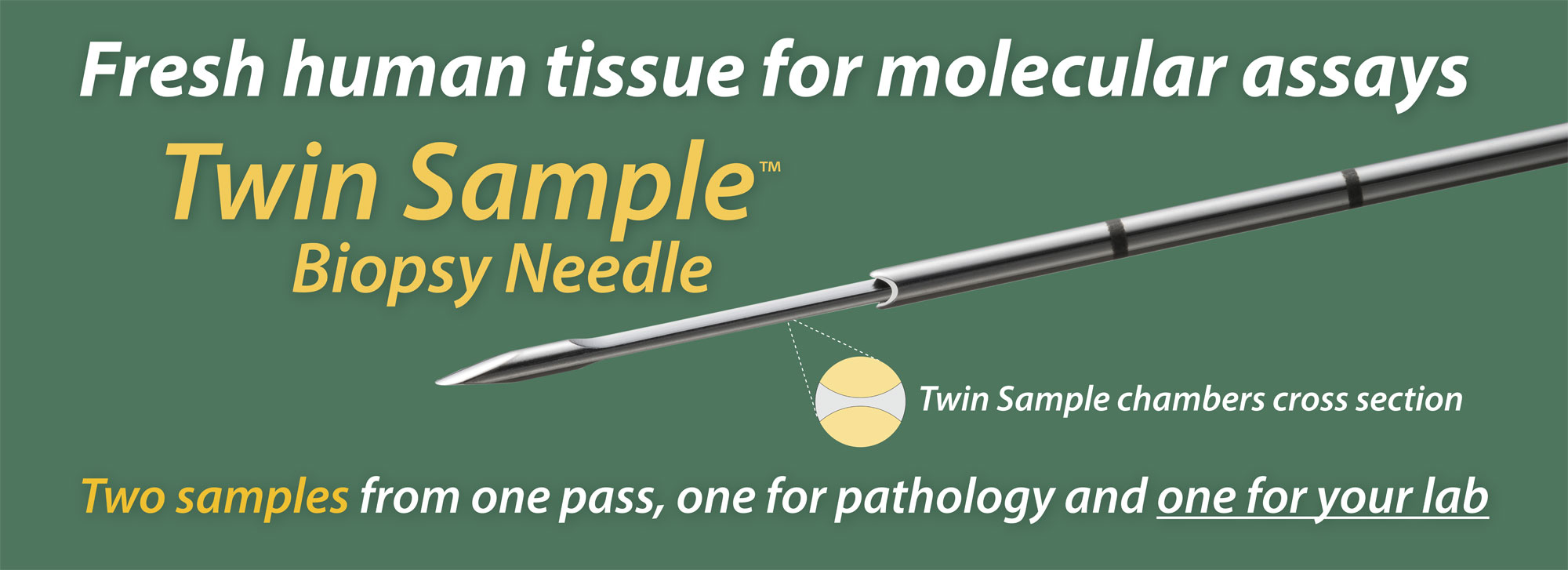
The Twin Sample biopsy needle obtains two samples in one pass; one for pathology and one for molecular assays. The two samples share a surface, as the two halves of a cylinder of tissue about one mm across. The pathology report informs the molecular analysis, since the percentage tumor cells affects the depth of coverage of sequencing.
Today interventional radiologists are asked to obtain up to 8 separate samples from the same tumor, but these blind biopsy samples are not the same as the first sample which goes to pathology. We know little about them. They are preserved in different ways, go to different departments and are extracted by different people on different machinery and reagents. Results may not be comparable and conflicting results add to the confusion of genomic medicine.
The Best Practices recommended by the NCI for biospecimens starts with the sentence: “The aim of every biospecimen resource should be to collect, maintain, and disseminate the highest quality biospecimens, based on the intended research use.”1 The mantra for diagnostics practice methodology is “fit for purpose,” so that the specimen is collected and handled to provide a reproducible and informative diagnosis. These methods need to be validated as “fit for purpose” and then standardized to ensure comparability.
The Right Sample: One sample is best, especially if many tests are contemplated, to reduce between-sample variance. One sample of fresh tissue is sufficient for all tests contemplated in precision medicine. This sample is then processed in one laboratory, the DNA, RNA and protein tested for quality once, aliquoted and distributed to multiple laboratories, along with the findings from the pathology report. A typical biopsy sample of 3 to 5 mg of fresh frozen tissue will yield between 3 to 5 µg of both DNA and total RNA, more than enough to perform several assays. Testing may involve a series of assays; is there enough DNA and RNA remaining to perform these additional tests? In this ideal scenario, no further biopsies will be needed. Everyone would be testing the same sample which has been characterized and processed properly. For targeted treatment, the patient's fate rests on the quality of this sample and its analysis, which changes the risk-benefit assessment.
The right biopsy: The Twin Sample biopsy needle delivers two matched samples in one pass. Only one good pass is needed. One of the two samples, for molecular analysis, should be processed immediately while the second sample, for pathology, receives Rapid On-Site Assessment (ROSE). If this sample is adequate, the procedure is complete; if it is inadequate, the biopsy is repeated. Sometimes the tumor cannot be sampled properly with a cutting needle, such as if it is lytic or necrotic. Other strategies may be implemented at that point.
A short 14G introducer needle is useful for use with the Twin Sample biopsy needle, so that a pneumothorax can be aspirated, a clotting substance delivered or to prevent tumor seeding. Because one pass will suffice for most procedures, the introducer does not have to be placed all the way to the tumor. The Twin Sample needle has ultrasound markings and obtaining a sample under ultrasound guidance is a classic procedure for accessible tumors.
The right team for implementation: Many reports stress the need for a team approach to obtain and analyze the tissue sample for precision medicine. The National Academies of Science have just issued an authoritative recommendation, which states:
The complexity involved in consistently obtaining adequate specimens requires communication throughout the entire health care team, including clinicians, surgeons, radiologists, and laboratory professionals, as well as consideration of the unique clinical conditions of each patient. 2
Recently the NCI-MATCH trial reported that 13% of biopsy samples were inadequate for testing when received at the central laboratory, despite the explicit instructions for taking biopsies.3 This trial called for four blind biopsies per tumor, and some centers were not delivering sufficient biopsies, as well as that some biopsies were inadequate for testing, primarily because they did not contain tumor tissue. The question of whether the blind biopsies gave conflicting results was not addressed in the summary of the trial pause.
Technology like the Twin Sample biopsy needle and better transport media for fresh tissue have a role to play in obtaining and testing the Right Sample, but they cannot replace teamwork, SOP's and standards. Getting the Right Sample deserves the full attention of the team and the institution, as the purpose of precision medicine can only be accomplished using the Right Sample. Considering the time and expense of the workup it will receive, and the interests of the patient, the Right Sample deserves that attention.
We hope this new approach will bring a new attitude and better quality tissue needed for precision medicine. We hope it will reduce the load on the interventional radiologist, on the lab processing the tissue and on the researchers running molecular assays and trying to explain variance when they have in fact been testing different samples. It will probably reduce cost and increase throughput for these specialties.
I look forward to hearing from you and want input on this approach.
Thank you very much.
Sincerely yours,
Paul T. Wegener
1 http://biospecimens.cancer.gov/bestpractices/to/bcpsrd.asp accessed 23 March 2016.
2 National Academies of Sciences, Engineering, and Medicine. 2016. Biomarker tests for molecularly targeted therapies: Key to unlocking precision medicine. Washington, DC: The National Academies Press. doi: 10.17226/21860
3 http://ecog-acrin.org/nci-match-eay131 accessed 23 March 2016